Connective Tissues
The Connective tissue is formed by
Cells and Extracellular matrix
(Intercellular matrix)
Extracellular Matrix
Function
- Stabilization of tissue structure.
- Regulation cell behavior
- Survival, development, migration, proliferation.
- Membrane filtration barrier (glomerules).
- Exchange of different metabolites, ions and water.
- Reparation function.
- Immune processes.
- Participation in inflammation.
Cells of Connective Tissue
- Fibroblasts
- Chondroblasts ( cartilage ).
- Osteoblasts ( bone ).
- Odontoblasts ( tooth ).
These cells synthesize extracellular matrix.
Parts of the extracellular matrix
FIBRILLAR PROTEINS ( collagen, elastin )
- Insoluble in water, no hydratation
GLYCOPROTEINS ( example : fibronectin, laminin ).
GLYCOSAMINOGLYCANS AND PROTEOGLYCANS
- Soluble in water, easily hydratated.
Extracellular matrix
Fibrillary Proteins :
- Structural Proteins
- Collagen – Firmness
- Elastin – elasticity
COLLAGENS
The most abundant proteins in mammals. They form approximately 25% of all body proteins.
Collagenum
Gr; kolla glue;
Gr; gennao constitute
By boiling collagen is denatured to a colloid solution (gelatine). From the non- purified collagen the glue arises.
- Main protein of the extracellular matrix.
- Component of tendons, cartilages, bones, and teeth ( dentin and cement), skin and vessels.
Properties
- Fibrillary proteins.
- Non soluble ( Glyco-) proteins.
- HIGH STRENGTH, BUT ALSO SUPPLENESS.
Structure of Collagen
Collagen has a Characteristic amino acid composition and their specific sequence.
Primary Structure
- Characteristic AA Composition
- Characteristic AA Sequence
Mature collagen contains no tryptophan and almost no cysteine- from the nutritional point of view not fully valuable protein.
Characteristic AA Composition
- Fundamental amino acids
- Glycine 33 % (x Hb 4 %)
- Proline 13 % (x Hb 5%)
- High content
- Derived amino acids
- 4- Hydroxyproline 9 % (x Hb 0%)
- 5- Hydroxylysine 0.6 %(x Hb 0%)
- Characteristic for collagen
- Origin by post translational modification

Read this topic also Overview of Cell injury and it’s Mechanic
Characteristic AA Sequence
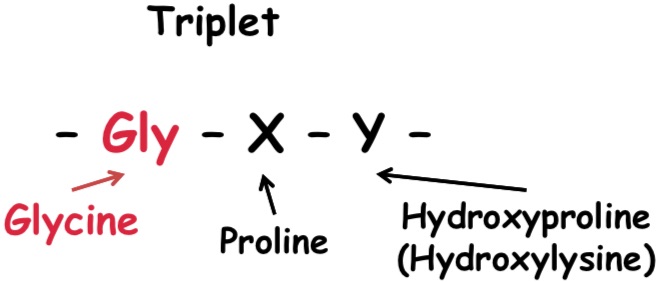
- Every third AA is GLYCINE.
- On the next position frequently PROLINE.
- On the third position frequently hydroxyproline, ev. Hydroxylysine.
Example of AA sequence of a part of the polypeptide chain
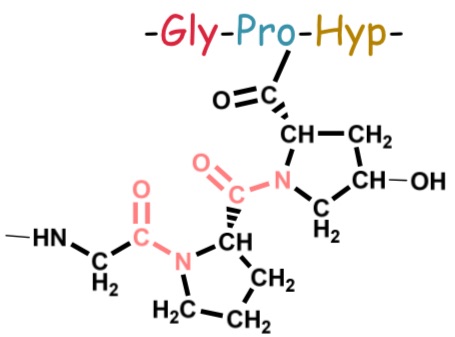
Secondary structure of collagen
Comparison of collagen helix to the alpha helix, which represents the most common secondary structure in proteins.
Collagen helix
- Levorotatory helix
- Steeper rising
- 3.3 AA / turn
- Intrachain hydrogen bonds not present
- Proline prevents formation of alpha-helix or beta- pleated sheet
Alpha- helix (the most common secondary structure in proteins)
- Dextrorotatory helix
- Gradual rising
- 3.6 AA / turn
- Stabilization by intrachain hydrogen bonds
Triple helix
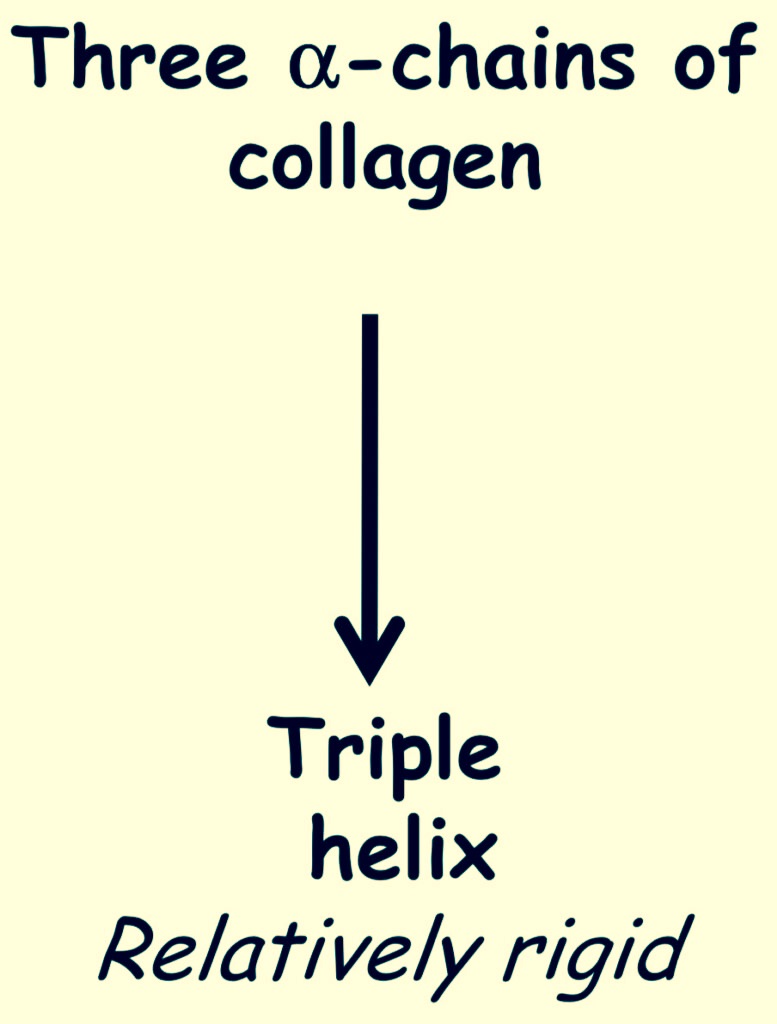
This structure is responsible for the tensile strenght.
The origin is dependent on the oddness of the primary structure
- High presence of Glycine
- Smallest amino-acid, no side chain (only -H)
- Placed in the centre of triple helix, where no space is available
- Close contact between the chains
Triple helix is stabilized by hydrogen bonds between each peptide bond -NH group of glycine and C=O group of the peptide bond of the adjacent polypeptide chain.
Leave a Reply